
-523.png)
With each side you twist, the cube tends towards greater disorder.StudySmarter Originals Each time you randomly twist the cube, you increase the number of possible configurations that your cube could take, decrease the chance of landing upon that perfectly solved arrangement, and get more and more disordered. But it is more likely that you will rotate a different side and disrupt the order even more. The second time you twist it, you might undo your first move and restore the cube to its original, perfectly solved arrangement. The first time you twist it, you disrupt the order. It starts off ordered - each face contains just one colour. However, when you break them down, they start to make a little more sense.
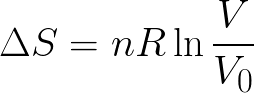
In the introduction to this article, we gave you one definition of entropy.Įntropy (S) is the number of possible ways that particles and their energy can be distributed in a system.
#Change in entropy formula free#
You'll find out how entropy, enthalpy, and temperature determine the feasibility of a reaction through a value known as G ibbs free energy. Finally, we'll explore the second law of thermodynamics and feasible reactions.We'll then look at entropy changes, and you'll be able to practice calculating enthalpy changes of reaction.We'll start by learning the definition of entropy and its units.This article is about entropy in physical chemistry.This idea of a neat arrangement spreading out into total chaos is a good starting point for entropy: a measure of disorder in a thermodynamic system. Under random action, you could say that the faces of the cube have gone from ordered and exact to a random configuration. What are the chances that it is still perfectly solved after twisting it around blindly for a couple of minutes? They're pretty low! Instead, it is quite likely that your cube isn't perfectly solved - the faces all contain a mixture of different colours. The cube could now have all sorts of possible arrangements. Take it into your hands, shut your eyes, and twist the sides around randomly a few times. Imagine a 2x2 Rubik's cube, solved so that each face contains just one colour. Reaction Quotient and Le Chatelier's Principle.Elemental Composition of Pure Substances.Application of Le Chatelier's Principle.

Intramolecular Force and Potential Energy.Variable Oxidation State of Transition Elements.Transition Metal Ions in Aqueous Solution.


 0 kommentar(er)
0 kommentar(er)
